December 3, 2023
KCR’s Son KT Rama Rao Leads By Over…
December 3, 2023
Chhindwara MP Election Result 2023: Congress stalwart Kamal…
November 28, 2023
Google Honours Chilean Javelin Athlete, Marlene Ahrens With…
November 11, 2023
A name is trending on the web and gaining people’s…










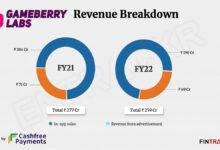


As robots become increasingly central to industrial operations across the…